Which Best Describes an Oxidizing Agent
Which best describes the oxidizing agent in this reaction. What reacts with carboxylic acids to make an ester.
What Is An Oxidizing Agent Definition From Corrosionpedia
Which best describes the oxidizing agent in this reaction.

. One of the most effective oxidizers known is hydrogen peroxide stronger than chlorine chlorine dioxide and potassium permanganate. CO2In this case oxygen would be the oxidizing agent as it. The oxidation state of the substance gets reduced during reduction.
A A substance that is reduced and causes oxidation of another substance. Fluorine is the best oxidising agent with the highest positive electrode potential value. Which best identifies why the rusting of an iron nail in the presence of water and oxygen is an oxidation-reduction reaction.
D A substance that reacts with oxygen. A good oxidizing agent is a metal in a high oxidation state such as Mn7 Oe. C A substance that is oxidized.
Reduction reaction is defined as the reaction in which a substance gains electrons. An example of a good oxidizing agent is an alkali metal such as Na. An oxidizing agent is a reactant that removes electrons from other reactants during a redox reaction.
Common examples of oxidizing agents include halogens such as chlorine and fluorine oxygen and hydrogen peroxide H 2 O 2. An oxidizing agent often referred to as an oxidizer or an oxidant is a chemical species that tends to oxidize other substances ie. Oxidation agent is normally on its higher oxidation state.
Cl2aq 2Braq 2Claq Br2aq Chlorine Cl is the oxidizing agent because it gains an electron. An oxidizing agent loses electrons. Which is Strongest Oxidizing Agent.
The substance N which itself gets reduced oxidizes other and is called as oxidizing agent. Which best describes an oxidizing agent. Which of the following statements best describes an oxidizing agent.
Which best describes the oxidizing agent in this reaction. Cause an increase in the oxidation state of the substance by making it lose electrons. Cl2aq 2Brmc003-1jpgaq mc003-2jpg 2Clmc003-3jpgaq Br2aq c.
And by catalysis with reactivity second only to fluorine H 2 O 2 can be transformed into hydroxyl radicals -OH. C A substance that is oxidized and causes reduction of another substance. A reactant that undergoes reduction Hydrogen fuel cells that operate at lower temperatures use more expensive platinum electrode catalysts.
Question 7 Which of the following best describes an oxidizing agent. Cl2aq 2Braq 2Claq Br2aq Chlorine Cl is the oxidizing agent because it gains an electron. The oxidation agent in a chemical reaction is the substance that is reduced because it gains electronsOxidizing agent also known as oxidant gains electrons and it is reduced during chemical reaction.
The strongest oxidizing agent in the list is F_2 followed by H_2O_2 and so on down to the weakest oxidizing agent Li. C O2 -. Whereas oxidation state of Br is changing from -2 to -3 this means Br is gaining electrons therefore it is an oxidizing agent.
The gainof oxygen loss of hydrogen or loss of electrons. Oxidizing is defined as. Besides which describes the oxidizing agent in a chemical reaction.
An oxidizing agent causes another. The substance M which itself gets oxidized reduces other and is called as reducing agent. The oxidation number of an oxidizing agent increases.
Oxidizing is defined as. Are bonds broken during phase change. Which best describes the oxidizing agent in this reaction.
Click to see full answer. B A substance that increases the number of oxygen atoms bonded. Chlorine Cl is the oxidizing agent because it gains an electron.

How To Find The Oxidizing And Reducing Agent Youtube

Solved Question 17 4 Pts Select All Of The Statements That Chegg Com
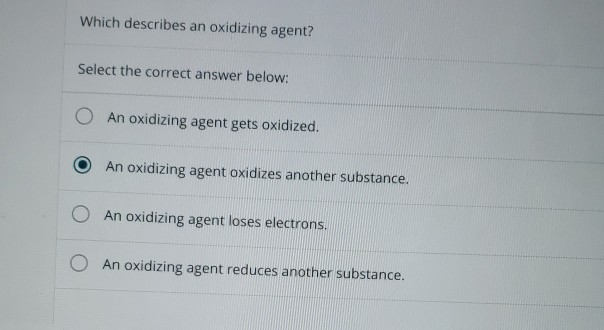
Solved Which Describes An Oxidizing Agent Select The Chegg Com

Difference Between Oxidation And Reduction Oxidizing Agent And Reducing Agent Oxidation State Oxidation State Reducing Agent Chemistry

Which Of The Following Statements Is True Of Retargeting In 2022 Retargeting True Relationship Management

Study Guide Chapter 6 Oxidation

If Both Statement I And Statement Ii Are True And Statement Ii Is The Correct Explanation Of Statement I
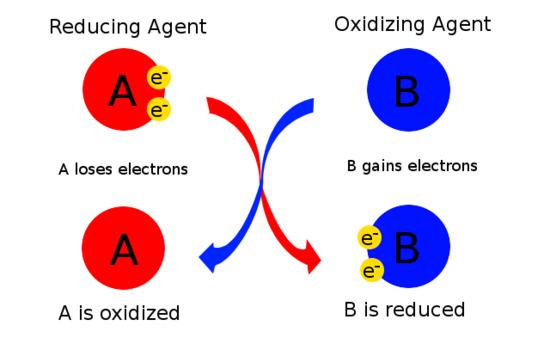
8 2 Oxidizing And Reducing Agents Chemistry Libretexts

Lesson Explainer Oxidization And Reduction Nagwa
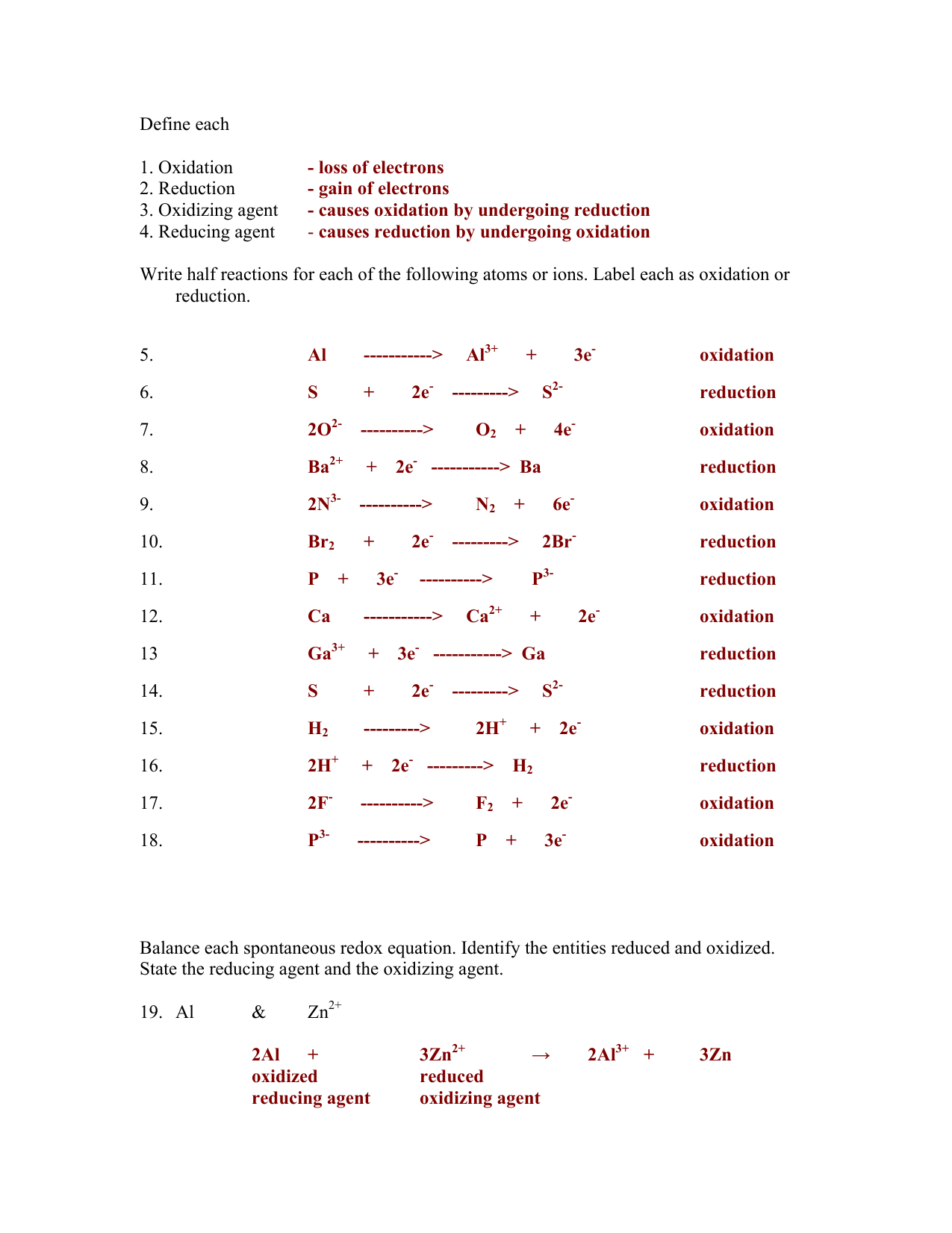
Workbook Oxidation Reduction Key

Solved Which Phrase Describes An Oxidizing Agent 1 Point Chegg Com

Oneclass Specify Which Of The Following Are Oxidation Reduction Reactions And If It Is Identify Th

Which Substance Is The Oxidizing Agent In This Reaction 2cuo C 2cu Co2 In 2022 Oxidizing Agent Substances Agents

Lesson Explainer Oxidization And Reduction Nagwa

Solved What Is The Oxidizing Agent Below F2 Aq 2 Nal Aq Chegg Com

Solved Question 7 Which Of The Following Best Describes An Chegg Com
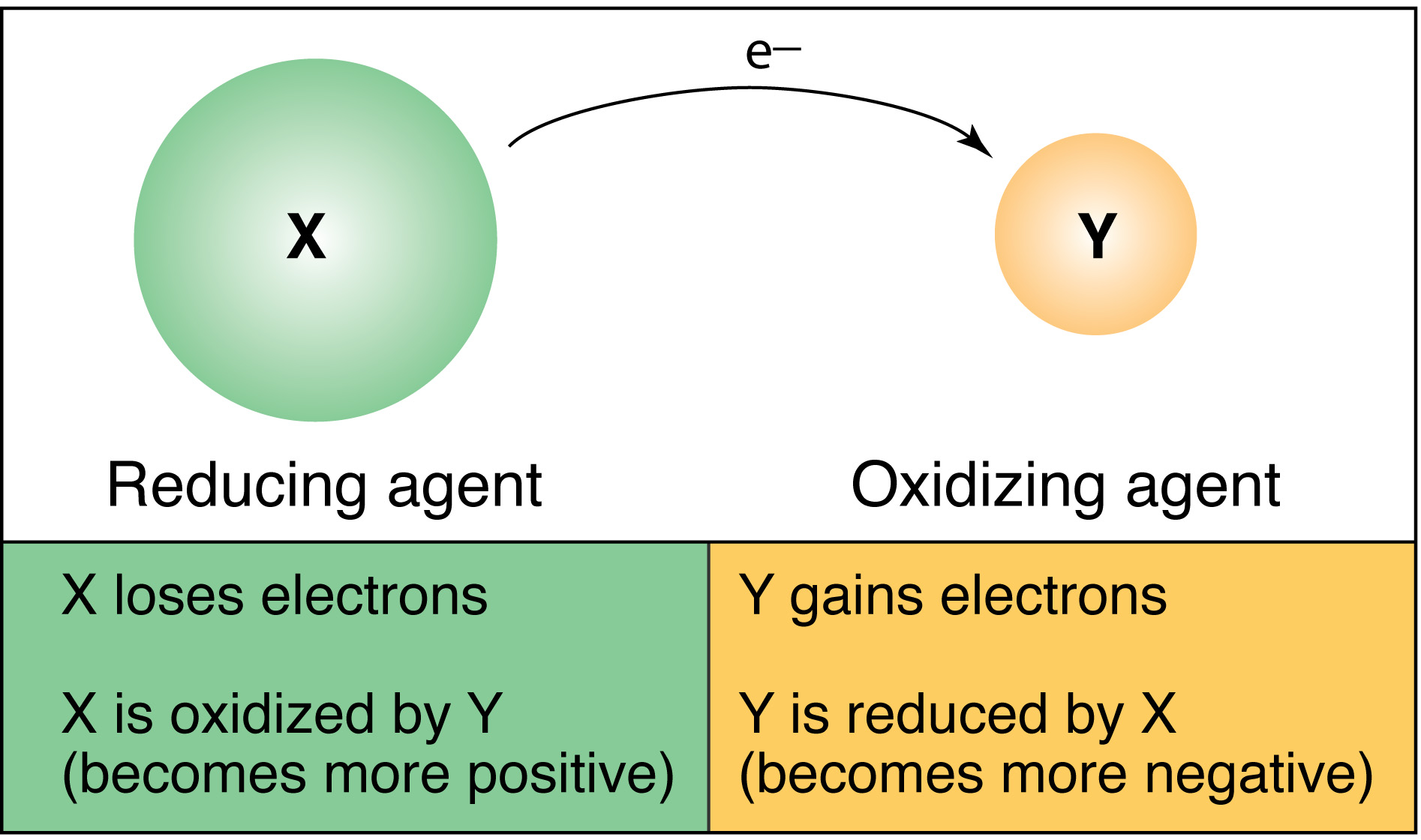

Comments
Post a Comment